From Wikipedia, the free encyclopedia
All living organisms are dependent on three types of very large molecules for essentially all of their biological functions. These molecules are DNA, RNA and proteins, and are classified as biological macromolecules.[1] Without DNA, RNA and proteins, no known forms of life could exist. This is because each molecule plays an indispensable role in biology.[2] The simple summary is that DNA makes RNA, and then RNA makes proteins.
DNA is an informational macromolecule that encodes the complete set of instructions (the genome) that are required to assemble, maintain, and reproduce every living organism.[3]
Proteins are responsible for catalyzing the myriad biochemical reactions that are required to provide food and energy for every organism, and for all forms of movement. In addition proteins carry out all of the other functions of any given organism, for example photosynthesis, or, for example in animals, neural function, vision, and structure (skin, tendons, exoskeleton, etc.).[4]
RNA is multifunctional, its primary responsibility is to make proteins, according to the instructions encoded within a cell’s DNA. They control and regulate many aspects of protein synthesis in eukaryotes.
Contents
- 1 Comparison of DNA, RNA and proteins
- 2 Common structural features of DNA, RNA and proteins
- 3 Divergent structural features
- 4 Why DNA is best for encoding genetic information
- 5 Why proteins are best for catalyzing biological reactions
- 6 Why RNA is multifunctional
- 7 DNA, RNA and proteins in molecular evolution
- 8 See also
- 9 References
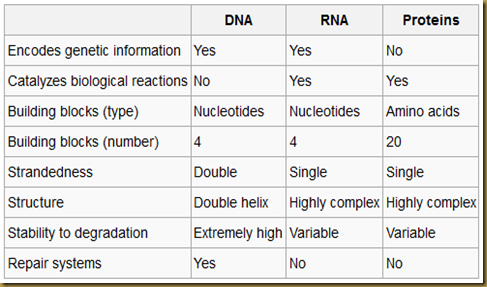
Comparison of DNA, RNA and proteins
Common structural features of DNA, RNA and proteins
While many typical cellular molecules (for example sugars and fats) contain tens, or rarely hundreds, DNA, RNA and proteins are typically composed of thousands of atoms (millions for most DNA molecules).
DNA, RNA and proteins are all polymers, long molecules that consist of a repeating structure of related building blocks (also termed monomers; nucleotides in the case of DNA and RNA, amino acids in the case of proteins). In general, DNA, RNA and proteins are all unbranched polymers, and so can be represented in the form of a string. Indeed, they can be viewed as a string of beads, with each bead representing a single nucleotide or amino acid monomer linked together through covalent chemical bonds into a very long chain.
In most cases, the monomers within the chain have a strong propensity to interact with other amino acids or nucleotides. In DNA and RNA, this can take the form of Watson-Crick base pairs (G-C and A-T or A-U), although many more complicated interactions can and do occur.
Divergent structural features
Because of the double-stranded nature of DNA, essentially all of the nucleotides take the form of Watson-Crick pairs between nucleotides on the two complementary strands of the double helix.
In contrast, both RNA and proteins are normally single-stranded. Therefore, they are not constrained by the regular geometry of the DNA double helix, and can and do fold into a vast number of complex three-dimensional shapes. These different shapes are responsible for many of the common properties of RNA and proteins, including the formation of specific binding pockets, and the ability to catalyze biochemical reactions.
Why DNA is best for encoding genetic information
DNA and RNA are both capable of encoding genetic information, because there are biochemical mechanisms which read the information coded within a DNA or RNA sequence and use it to generate a specified protein. On the other hand, the sequence information of a protein molecule is not used by cells to functionally encode genetic information.
DNA has three primary attributes that allow it to be far better than RNA at encoding genetic information. First, it is normally double-stranded, so that there are a minimum of two copies of the information encoding each gene in every cell. Second, DNA has a much greater stability against breakdown than does RNA, an attribute primarily associated with the absence of the 2'-hydroxyl group within every nucleotide of DNA. Third, highly sophisticated DNA surveillance and repair systems are present which monitor damage to the DNA and repair the sequence when necessary. Analogous systems have not evolved for repairing damaged RNA molecules.
Why proteins are best for catalyzing biological reactions
The single-stranded nature of protein molecules, together with their composition of 20 or more different amino acid building blocks, allows them to fold in to a vast number of different three-dimensional shapes, while providing binding pockets through which they can specifically interact with all manner of molecules. In addition, the chemical diversity of the different amino acids, together with different chemical environments afforded by local 3D structure, enables many proteins to act as enzymes, catalyzing a wide range of specific biochemical transformations within cells. In addition, proteins have evolved the ability to bind a wide range of cofactors and coenzymes, smaller molecules that can endow the protein with specific activities beyond those associated with the polypeptide chain alone.
Why RNA is multifunctional
RNA encodes genetic information that can be translated into the amino acid sequence of proteins, as evidenced by the messenger RNA molecules present within every cell, and the RNA genomes of a large number of viruses. The single-stranded nature of RNA, together with tendency for rapid breakdown and a lack of repair systems means that RNA is not so well suited for the long-term storage of genetic information as is DNA.
In addition, RNA is a single-stranded polymer that can, like proteins, fold into a very large number of three-dimensional structures. Some of these structures provide binding sites for other molecules and chemically-active centers that can catalyze specific chemical reactions on those bound molecules. The limited number of different building blocks of RNA (4 nucleotides vs >20 amino acids in proteins), together with their lack of chemical diversity, results in catalytic RNA (ribozymes) being generally less-effective catalysts than proteins for most biological reactions.
References
http://en.wikipedia.org/wiki/DNA,_RNA_and_proteins:_The_three_essential_macromolecules_of_life